Introduction
Materials and Methods
1. Plant Materials and Growth Conditions
2. Runner Training
3. Measurements of the Growth and Morphological Parameters
4. Measurements of Contents of Soluble Sugar and Starch
5. Data Collection and Statistical Analysis
Results and Discussion
Introduction
Strawberry (Fragaria × ananassa Duch.) is a herbaceous perennial crop that belongs to the Rosaceae family. It has high economical and nutritive values (El-Deeb and Mohamed, 2018) and is widely cultivated in temperate and subtropical regions of the world (Sharma and Thakur, 2008). Asexual propagation through runners, division of the mother ramet, tissue culture, and seed propagation are the four main ways of reproducing strawberry plants (Nyman and Wallin, 1992; Gaston et al., 2013; Guan et al., 2019). The horizontally elongated runners that grow along the ground consist of internodes and runner plants at the even-numbered nodes. Nowadays, propagation through runner plants is the easiest and most commonly used method in commercial farms (Caruana et al., 2018). Runner plants are reproduced true to type by runner propagation and maintain the elite genotypes of their mother plants. Thus, runner production carries a great agricultural value and commercial profit. For short- day genotype and seasonally flowering woodland strawberry (F. vesca), the temperature and photoperiod affect the runner formation (Smeets, 1982; Heide and Sønsteby, 2007; Hytönen, 2009). Furthermore, runner formation is also affected by the light intensity (Kim et al., 2010; Zheng et al., 2019), light quality (Black et al., 2005; Wu et al., 2009Takeda et al., 2010), and phytohormones, such as cytokinin (Pipattanawong et al., 1996), auxin (Qiu et al., 2019), and GA (Thompson and Guttridge, 1959; Dale et al., 1996). Axillary buds keep dormant or evolve into runners depending on the bud activity. Auxin and cytokinin play a reversely synergic role in coordinating the dormancy and outgrowth of axillary buds (Qiu et al., 2019). Decapitating the apical bud to release apical dominance (Neri et al., 2003) and applying cytokinin (Liu et al., 2019) could influence the number of runners and the length of internodes in strawberry plants, as previous studies have shown.
The aerial parts of a plant usually grow in the opposite direction of gravity. However, the growth of vines and runners always follows gravitropism (Givnish, 1995). Manually adjusting the branch orientation to horizontal and downward directions could influence the number of flowers and vegetative growth in fruit trees such as apple, pear, cherry, and guava (Lauri et al., 1998; Ito et al., 1999Han et al., 2007; Bagchi et al., 2008). Additionally, shoot bending is also applied in ornamental plant production, a technique that has proven to play an important role in regulating the biochemical contents, water balance, and photosynthesis (Bagchi et al., 2008; Liu and Chang, 2011; ).
During the commercial vegetative propagation using runner plants, excessive extension growth of runner internodes deteriorates the growth conditions and thereby reduces the productivity of mother plants. Suppressing the extension growth of internodes and promoting runner plant production by runners are the key to increasing the propagation efficiency. This study was conducted to investigate the effects of the runner training angle (RTA) on the length of internodes and the number of runners produced in strawberry ‘Seolhyang’ and ‘Maehyang’.
Materials and Methods
1. Plant Materials and Growth Conditions
The experiment was conducted in a glasshouse at Gyeongsang National University in the Republic of Korea. The glasshouse had 29/20℃ day/night temperatures, an average light intensity of 450 μmol·m-2·s-1 PPFD coming from the sun, and a natural photoperiod of 12 hours. The widely cultivated strawberry cultivars ‘Seolhyang’ and ‘Maehyang’ were used in this study. Mother plants were obtained from a farm located in Sugok-myeon, Jinju, Gyeongsangnam-do, the Republic of Korea. After removing the excrescent leaves, ramets, and axillary buds, uniform mother plants with 3 mature leaves were transplanted into 10-centimeter pots (Green-100, Danong Co., Namyangju, Republic of Korea) filled with the BVB Medium (Bas Van Buuren Substrate, EN-12580, De Lier, The Netherlands).
2. Runner Training
After new runners formed, mother plants with a single runner approximately 5 cm in size were selected for the experiment. To guide the direction of the runner growth, the runners were fixed loosely on thin plastic sticks. Runners were trained to grow at an angle of 0° (vertically upward), 45°, 90° (horizontal), 135°, or 180° (hanging down) from the upward vertical axis as shown in Fig. 1. The training angle lasted for 30 days from May 25, 2019.
3. Measurements of the Growth and Morphological Parameters
The number and length of runners, number of runner plants, length of internodes, length and width of leaves, fresh and dry weights of runners, and runner diameter were measured after 30 days of the RTA treatments. The dry weights were measured after 48 hours of drying in an oven (FO-450M, Jeio Technology Co. Ltd., Daejeon, Republic of Korea) at 60 ℃.
4. Measurements of Contents of Soluble Sugar and Starch
Measurements of contents of soluble sugar and starch were done using modified methods described by Dubois et al. (1951). The 0.5 g leaf samples were frozen and ground in liquid nitrogen to a powder form. Then the samples were transferred to 50 mL centrifugal tubes and homogenized with 20 mL distilled water for 30 minutes in boiling water and shaking the centrifugal tubes once every 10 minutes for blending. The mixture was centrifuged for 10 minutes at 6,500 rpm after cooling down. The clear supernatant extracts and residues were used for measurements of contents of soluble sugar and starch, respectively. The supernatants were filtered and transferred to 50 mL volumetric flasks and diluted with distilled water to a volume. Three 15 mL centrifugal tubes containing 0.1 mL supernatant extracts of each treatment, 1.9 mL distilled water, 0.5 mL 2% anthrone ethylacetate, and 5 mL 98% H2SO4 were used for the chromogenic reaction. With the replacement of supernatant extracts to 0.1 mL distilled water, the same other reagents were added to one new centrifugal tube as the blank control. The tubes were placed in a boiling water bath for 10 minutes. The absorbance at 630 nm was recorded and content of soluble sugar was calculated by a standard curve. The residues were transferred to 50 mL centrifugal tubes and homogenized with 20 mL distilled water for 15 minutes in boiling water. The 2 mL 9.2 mol·L-1 HClO4 was added to each tube and the mixture was centrifuged for 10 minutes at 2,500 rpm after cooling down. The supernatants were filtered and transferred to 50 mL volumetric flasks and diluted with distilled water to a volume. The processes of the chromogenic reaction were conducted as the same as the measurement of content of soluble sugar. The absorbance at 485 nm was recorded and content of starch was calculated by a standard curve.
5. Data Collection and Statistical Analysis
One-way analysis of variance and a Duncan multiple range test (p ≤ 0.05) were used to evaluate the data with the SAS statistical software package (SAS Institute Inc., NC, USA). OriginPro software version 9.0 (OriginLab Co., Northampton, MA, USA) was used to produce the graphs.
Results and Discussion
For both ‘Seolhyang’ and ‘Maehyang’, the RTA significantly affected number and length of the runners. A RTA of 135º or 180º decreased number and length of the runners (Figs. 2A, B). Number of runners increased as the RTA increased from 45° to 90° in ‘Seolhyang’. Under the 135° RTA, smallest number of runners was achieved, followed by those under the180° RTA (Fig. 2A). Number of runners per plant was as much as approximately two times higher in the 90° RTA than the 135° RTA. In ‘Maehyang’, no difference in number of runners was observed in the 45°, 90°, and 135° RTAs. Plants in the 0° RTA had the greatest number of runners, followed by plants in the 180° RTA (Fig. 2A) in ‘Maehyang’. Besides, the shortest runner length was observed in the 180° RTA in both cultivars (Fig. 2B).
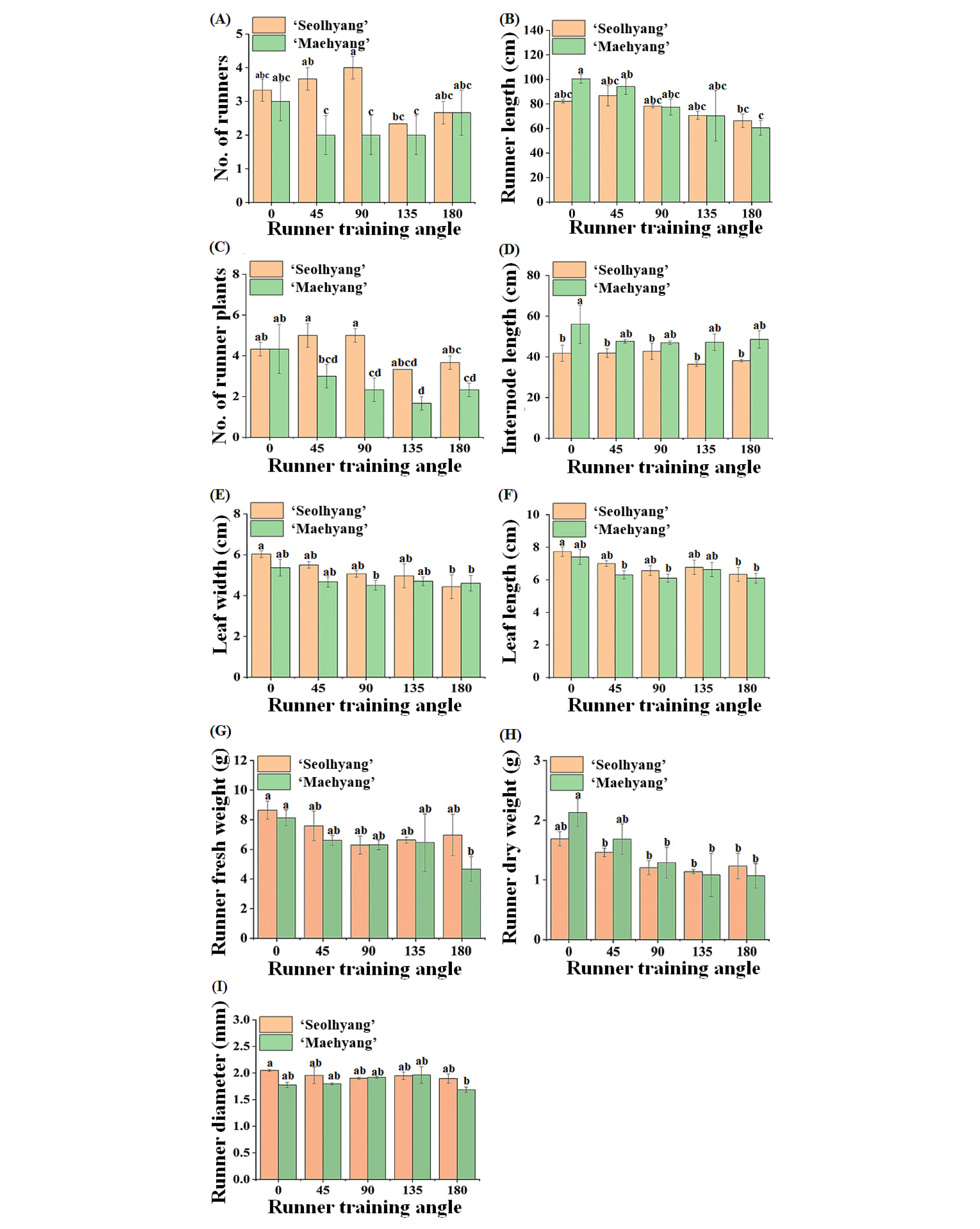
Fig. 2.
Effect of the runner training angle (RTA) on growth and development of runners in ‘Seolhyang’ and ‘Maehyang’ strawberries, measured at 30 days after the RTA setup. Vertical bars show standard errors of nine biological replicates (n=9). Lower case letters at p ≤ 0.05 indicate significant differences among treatments according to the Duncan test.
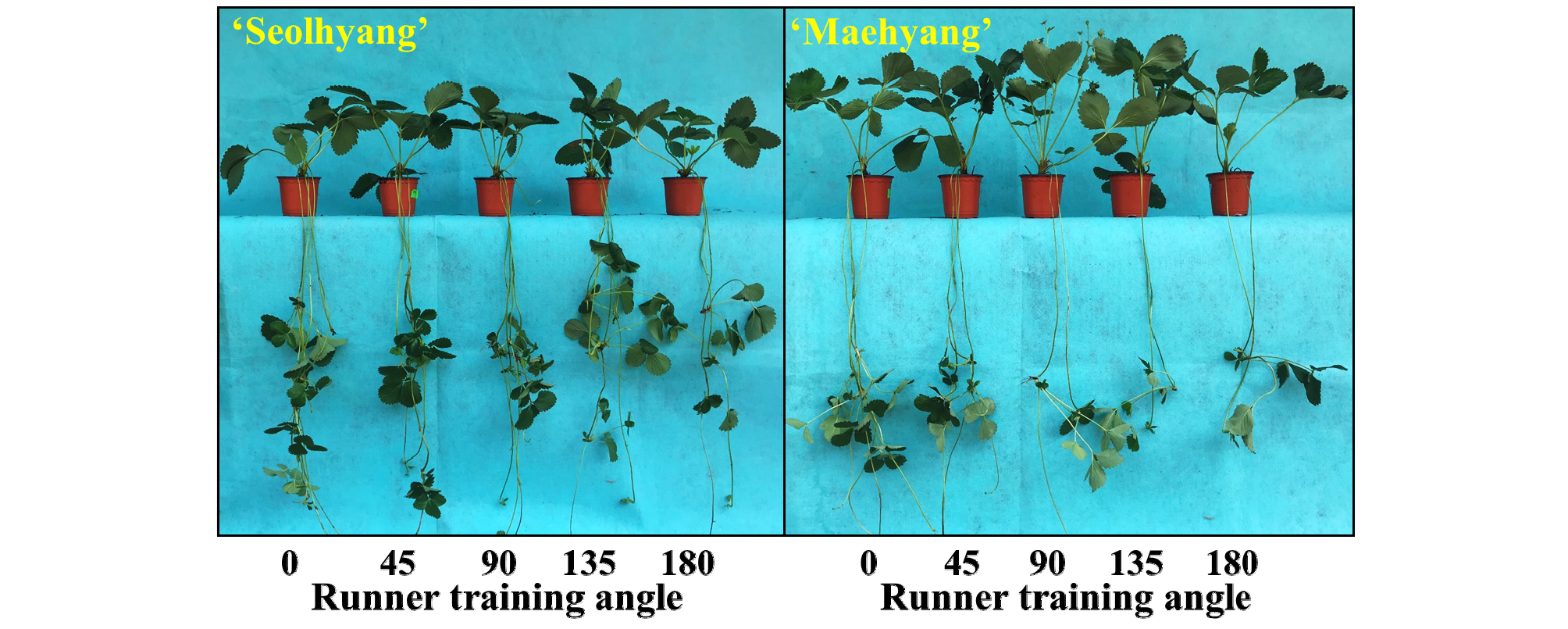
The RTA also affected the number of runner plants (or daughter plants) in both cultivars. The trend in number of runner plants in ‘Seolhyang’ in the 135° and 180° RTAs was similar to number of runners in ‘Seolhyang’. A RTA of 135º was effective in reducing number of runners, which was about 60% of that in the 90º RTA in ‘Seolhyang’ (Fig. 2C). Number of runners was slightly higher in the 180° as compared to 135° RTA (Fig. 2C). In ‘Maehyang’, number of runner plants decreased with the increasing RTA from 0° to 135°. The 180° RTA produced a larger number of runner plants as compared to the 135° RTA (Fig. 2C). The RTA also had a slight influence on the internode length. Internode length didn’t change dramatically in both cultivars. In ‘Maehyang’, plants in the 0° RTA had the longest internode length, which was approximately 20% longer than those in the other treatments (Fig. 2D). However, in Seolhyang’, plants in the 135° and 180° RTAs had approximately 14% shorter internode length than in other treatments (Fig. 2D). The width and length of leaves were negatively correlated with the RTA (Figs. 2E, F). In ‘Seolhyang’, leaf width and length were the greatest in the 0° RTA and the smallest in the 180° RTA, and there were no remarkable differences among the other treatments (Figs. 2E, F). For leaf width and length in ‘Maehyang’, although slightly greater values were observed in the 0° RTA, there were no significant statistical differences among treatments (Figs. 2E, F). The 0° RTA also gave the greatest runner fresh and dry weights in both ‘Seolhyang’ and ‘Maehyang’, and there were no significant differences in the other RTAs (Figs. 2G, H). The runner fresh and dry weights slightly increased in the 180º RTA in ‘Seolhyang’ as compared to the 135º RTA (Figs. 2G, H). The RTA did not seem to affect the runner diameter (Fig. 2I). Photographs of the representative ‘Seolhyang’ and ‘Maehyang’ plants showed that the RTA affected the morphology of the first internode of the runner in both cultivars (Fig. 3). As compared to a RTA of 135º, a RTA of 180º led to a higher number of runner plants produced, making it the most proliferative RTA.
Contents of the endogenous soluble sugar and starch were affected by the RTA (Fig. 4). In ‘Seolhyang’, content of soluble sugar increased in the 135° and 180° RTAs as compared to the 0° to 90° RTAs (Fig. 4A). The trend of content of starch was in the same as content of soluble sugar. The greatest content of starch was observed in the 135° and 180° RTAs (Fig. 4B). However, contents of soluble sugar and starch were not significantly influenced by the RTA in ‘Maehyang’ (Figs. 4A, B).
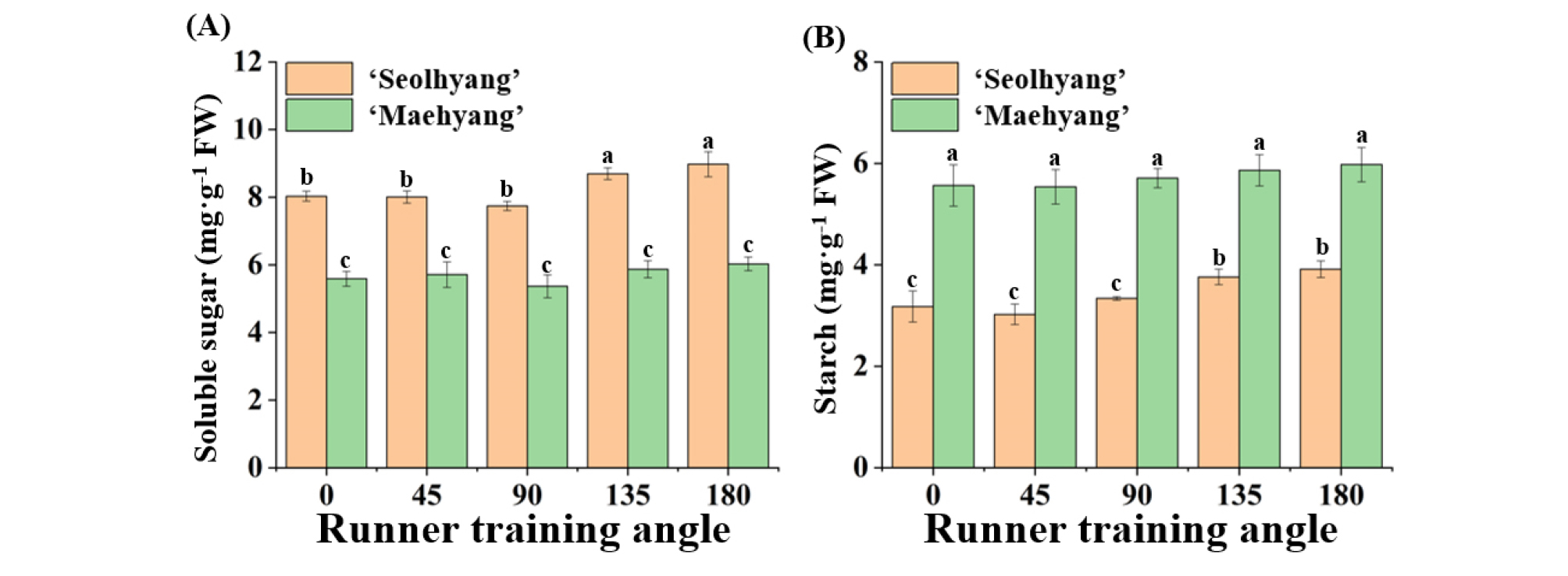
Fig. 4.
Effect of the runner training angle (RTA) on contents of soluble sugar (A) and starch (B) of ‘Seolhyang’ and ‘Maehyang’ strawberries, measured at 30 days after the RTA setup. Vertical bars show standard errors of three biological replicates (n=3). Lower case letters at p ≤ 0.05 indicate significant differences among treatments according to the Duncan test.
An earlier study has shown that a downward orientation of the shoot decreased shoot length, number of internodes, and total leaf area in Vitis vinifera (Schubert et al., 1999). The results of this study also showed that the RTA could influence the parameters related to growth and development of runners such as their number (Fig. 2A) and length (Fig. 2B), and number of runner plants (Fig. 2C), in ‘Seolhyang’. The altered hydraulic conductivity (kh) caused by the shoot orientation may play an important role in this regulation mechanism (Salleo et al., 1985; Schubert et al., 1999). In addition, research conducted with Pharbitis nil had shown that an inverted orientation of the shoot may lead to decreased fresh and dry weights of the shoot, and it was also observed in this study as the 0° RTA led to the greatest fresh and dry weights of the runners in both cultivars (Figs. 2G, H).
These effects of a horizontal or inverted orientation on the lateral bud and shoot growth, as well as radial stem expansion, were mediated by the reduced content of ethylene in Pharbitis nil (Prasad and Cline, 1987). Furthermore, it has already been demonstrated that adjusting the shoot angle from a vertical to horizontal position decreased the indole-3-acetic acid (IAA) content and IAA transport velocity in Japanese pear shoots (Ito et al., 2001). Bending the shoots resulted in increased ethylene production by the shoots and changed the ratio of floral and vegetative buds in apple (Sanyal and Bangerth, 1998). The released growth of the axillary bud from apical dominance was regulated by various environmental factors including gravity (Kitazawa et al., 2008). It is also supposed that gravity is involved in the RTA’s auxin-ethylene regulatory mechanism on the shoot growth (Prasad et al., 1987; Philosoph-Hadas et al., 2005). Strigolactones are a group of a newly-discovered plant hormone that act in the control of shoot branching and regulate the rice tiller angle through suppressing the auxin biosynthesis (Umehara et al., 2008; Sang et al., 2014). Additionally, zeatin-type cytokinin concentrations were up-regulated in buds in bent shoots, when compared to those in vertical shoots (Ito et al., 2001). Besides, the carbohydrate contents (fructose, glucose, sorbitol, sucrose, and starch) and activities of sugar metabolizing enzymes were negatively regulated by increased shoot angles (Ito et al., 2004). The rice LAZY1 (LA1) gene has been demonstrated to control the rice tiller angle through negative regulation of polar auxin transport (PAT) (Li et al., 2007). In Arabidopsis, AtLAZY1 takes part in the gravity-sensing processes and branch angle regulation (Yoshihara et al., 2013). In this study, contents of soluble sugar and starch were affected by the RTA in ‘Seolhyang’ (Figs. 4A, B). Except for auxin mentioned above, previous studies have shown that exogenous application of GAs (Qureshi et al., 2013) and ethephon (Liu et al., 2019) also has effects on the carbohydrate content and growth and development of runners. In future studies, it needs to explore the mechanism of phytohormones involved in the effect of the RTA on runners.
Different RTAs caused changes in parameters related to growth and development of runners and carbohydrate contents in this study. The RTA alteration may play an important role in the reproductive and vegetative growth of plants. However, studies on RTAs are mainly focused on woody and ornamental plants, and there has been no study on the effects of the RTA on strawberry until now. This study found that training runners to different angles affected the growth and development of runners in ‘Seolhyang’ and ‘Maehyang’ strawberry. Compared to a RTA of 135°, a RTA of 180° increased the number of runner plants produced, making it the most proliferative RTA. In future studies, the underlying regulation mechanism will be studied. The findings of this study may directly provide guidance to strawberry production.