Introduction
Hydroponics is a plant cultivation method in which individual crops are supplied with optimal concentrations of nutrients. It consists of two types of system, closed and open, depending on whether the nutrient solutions are reused or not. Recently, the number of facilities that recirculate and reuse hydroponic solutions has been increasing due to concerns over environmental pollution and cost (Rius-Ruiz et al., 2014; Jung et al., 2015). However, prior to their reuse, it is important to measure the concentrations of the nutrients within the hydroponic solutions.
The efficient monitoring of nutrients in hydroponic solutions using real-time and on-site methods would allow the accurate estimation of the required application loads of variable-rate fertilizer in agricultural fields and greenhouses. Current practices for monitoring ion concentrations in hydroponic solutions are typically based on the measurement of electrical conductivity (EC) and pH (Jung et al., 2015; Kim et al., 2013; Savvas and Manos, 1999). Using these methods, the quality of hydroponic solutions is maintained through the automatic supply of nutrient solutions in proportion to the reduction in the EC that results from the nutrient uptake of plants (Jung et al., 2019b). A problem with this practice is that EC measurements provide no information on individual ion concentrations, thereby limiting its ability to effectively replenish the specific ions essential to maximize crop growth (Savvas and Manos, 1999; Savvas 2002; Bamsey et al., 2012).
Many researchers have reported the use of ion-selective electrodes (ISEs) to measure changes in ion concentrations in nutrient solutions. ISE technology has several advantages over other analytical methods, such as its simplicity of use, direct measurement of the analyte, sensitivity over a wide concentration range, low cost, and portability (Bamsey et al., 2012; Rius-Ruiz et al., 2014; Cho et al., 2017; ; ). However, its measurement accuracy may be affected by signal drift and reduced sensitivity over time; thus, it requires the use of a computer-based measurement system that conducts automatic solution sampling and electrode calibration (Kim et al., 2013b). It has been reported that the use of two-point normalization consisting of sensitivity compensation followed by offset adjustment was effective in reducing the signal drift of electrodes for specific ions. Thus, an automated measurement system based on automatic sampling and calibration makes it possible to more accurately determine nutrient concentrations using ISEs (Dorneanu et al., 2005).
Several automatic measurement systems using PCs have been developed in previous studies, but the use of an embedded system based on microcomputers would be more advantageous for the design of an on-site nutrient analyzer with an array of sensors because the size and production cost of the system could be minimized while maintaining reliability and performance (Kim et al., 2017; Kim et al., 2017). At present, most commercial portable analyzers based on ISEs, such as pH meters, operate with an embedded system. Although an automatic embedded system for on-site use has been developed (Kim et al., 2017), it was only possible to measure NO3 concentration. Similarly, PC-based measurement systems in conjunction with two-point normalization used to quantify NO3, K, and Ca ions in hydroponics solutions have demonstrated an acceptable level of accuracy (Cho et al., 2017; Jung et al., 2015).
Phosphorus is known as an important element for crop growth because both its deficiency and excess can have deleterious effects (Choi et al., 2007). Therefore, the concentration of phosphate ions in nutrient solutions needs to be carefully monitored. However, there are some limitations to phosphate ion measurement in hydroponic solutions because phosphate ions exist in various forms depending on the pH. As a result, a measurement system for phosphate ions has not yet been established (Jung et al., 2019). Although previous studies (Kim et al., 2007) have measured the concentration of phosphate ions in soil and nutrient solutions using cobalt electrodes, continuous measurement has proven difficult (Kim et al., 2007; Jung et al., 2019, ). In particular, cobalt electrodes need to be calibrated frequently because the continuous measurement of hydroponic solutions leads to an oxidation reaction on the cobalt surface, resulting in lower measurement sensitivity. If these limitations of cobalt electrodes can be overcome in automated measurement systems, these systems can be used in the agricultural field.
The ultimate goal of this study was to develop an ISE- based embedded system with automatic sampling and calibration functions using a micro-control unit (MCU) board to measure in real-time. For this reason, the specific objectives of this study were 1) to build a prototype of the proposed ISE-based embedded monitoring system for hydroponic nutrients and 2) to investigate its feasibility for the real-time measurement of NO3, K, Ca, and P ions in hydroponic solutions.
Materials and Methods
To determine the concentrations of NO3, K, and Ca ions in solutions based on ISEs, three different PVC-based ion- selective membranes were prepared with the chemical compositions presented in Table 1. The NO3 and K membranes were fabricated following the processes reported in our previous studies (Kim et al., 2013a; Kim et al., 2013b; Kim et al., 2017). The chemical composition of the membrane for the Ca ISE was based on the methods described in previous studies (Schefer et al., 1986; Asadnia et al., 2017).
Table 1. Chemical composition of the NO3, K, and Ca ISE membranes used in the present study.
bwt% - percentage by weight
The tested PVC-based membranes were produced by dissolving each of the chemical mixtures in 2 mL of tetrahydrofuran (THF). When a mixture was completely dissolved, it was poured into a 23-mm diameter glass ring resting on a polished glass plate and then allowed to evaporate for 24 h at room temperature (20–25°C) to form a membrane film (0.25–0.35 mm in thickness). Membrane disks with a diameter of 2.5 mm were punched from the membrane film and attached to the ends of laboratory- produced plastic electrode bodies, 44 mm in length, using THF solvent (Kim et al., 2017). The NO3, K, and Ca ISEs were immersed in 0.01 M NaNO3 + 0.01 M NaCl, 0.01 M KCl, and 0.01 M CaCl2 as internal solutions, respectively. An Ag/AgCl electrode prepared by coating a 99% silver wire with a diameter of 1 mm with Ag/AgCl ink (ALS Co., Tokyo, Japan) was employed as the inner reference electrode. A double-junction electrode (Thermo Fisher Co., MA, USA) was utilized as the reference electrode.
To detect phosphate, cobalt electrodes 99.95% of purity were prepared according to the process reported in previous studies (Xiao et al., 1995; Kim et al., 2007; Jung et al., 2019). As shown in Fig. 1, the cobalt electrodes were fabricated from a 99.95% pure cobalt rod with a diameter of 5 mm (Sigma Aldrich Co., MO, USA), which was cut to a length of 8 mm and soldered to 1-mm copper wire. The length of the cobalt rod was increased from 5 mm to 8 mm compared to the cobalt electrodes used in previous studies to improve the measurement performance. The cobalt rod was inserted into a plastic electrode body with an external diameter of 6 mm and an internal diameter of 5 mm, and silicon was injected between the cobalt rod and the plastic body to seal it (Fig. 1c). In accordance with the methods described in a previous study (Kim et al., 2007), the cobalt electrodes were pre-treated to ensure stable measurement. The electrodes were first polished using, in sequence, 400, 1000, and 1500 grit emery sheets and then immersed in deionized water (DI) for about 20 min. They were then immersed in a low buffer solution of potassium hydrogen phthalate (KHP) containing no phosphate for 20 min. After this pre-treatment process, the electrodes were able to be used in subsequent experiments.
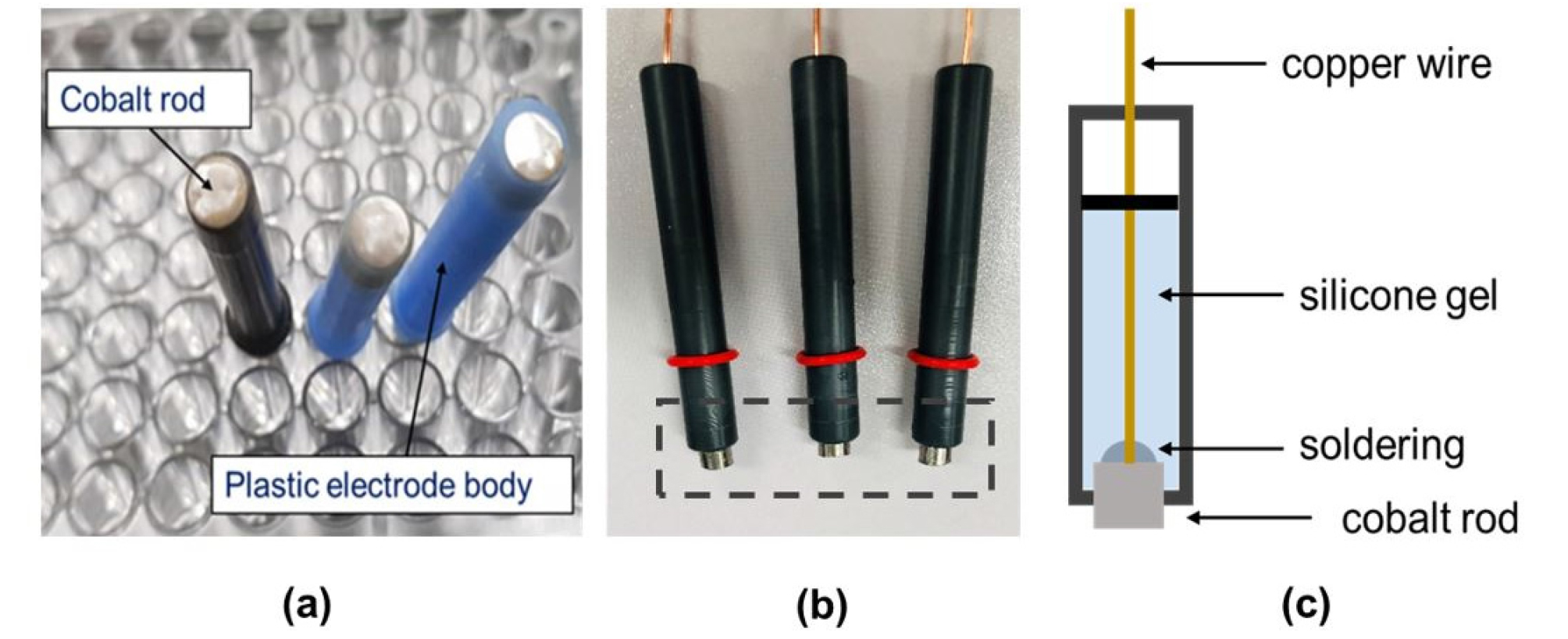
Fig. 1.
(a) Image of the cobalt electrodes used in a previous study (Jung et al., 2019). (b) Image of the modified cobalt electrodes. (c) Schematic diagram of a modified cobalt electrode.
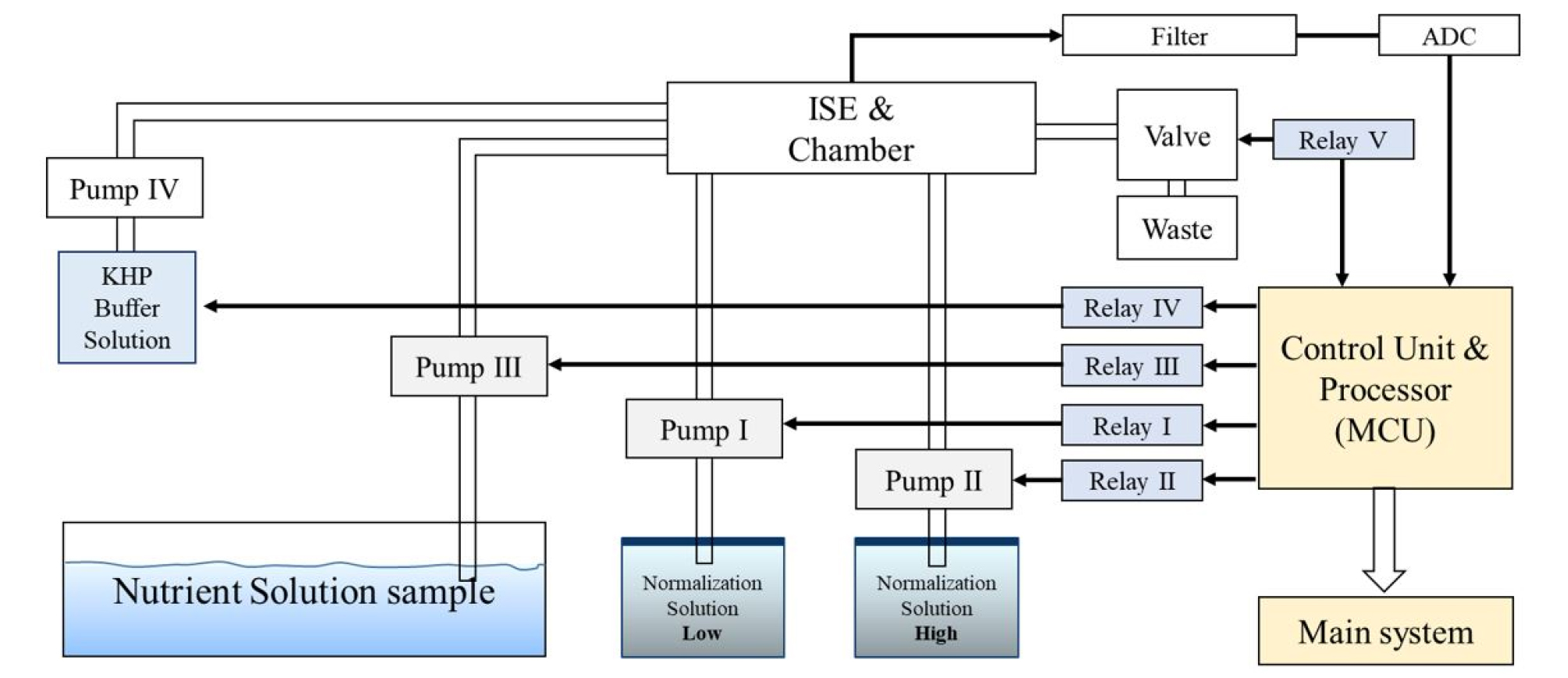
The prototype of the ISE-based embedded system proposed in this study consisted of a combination of six NO3, K, and Ca ISEs (two each), a double-junction reference electrode, a solution container, a sampling system with three pumps and solenoid valves, a signal processing circuit, and an Arduino Due board for data acquisition and system control (Fig. 2a). The system conducted automatic sampling and normalization by controlling the pumps and valves using the 8-channel relay shown in Fig. 2(b) to fill and empty the container. To measure the concentration of P ions, a container with KHP buffer solution was used to maintain the pH of the test sample. A block diagram that presents the components of the system and their connections is presented in Fig. 3.
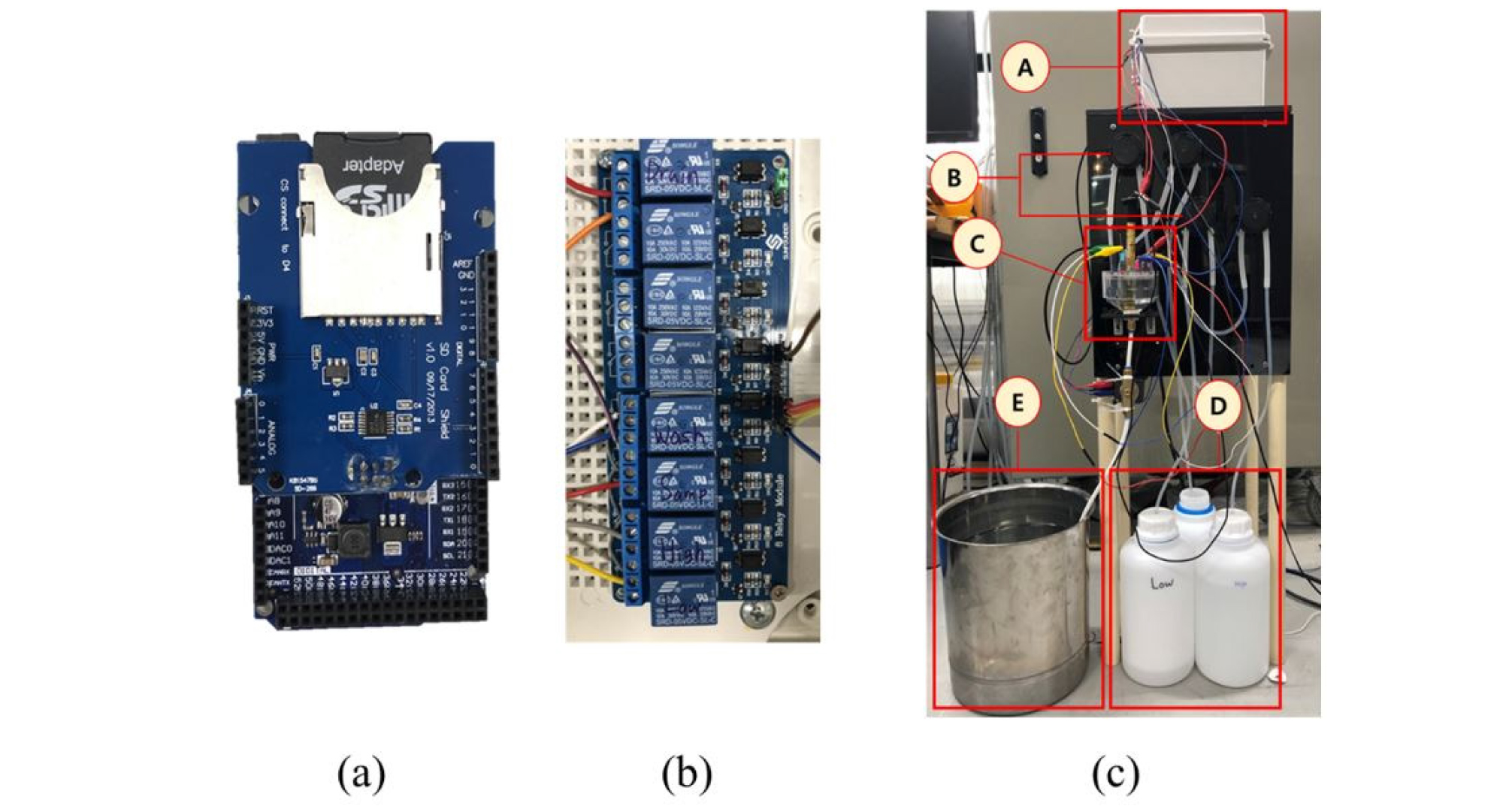
Fig. 2.
Images of (a) the Arduino Due board, (b) the 8-channel relay for pump and valve control, and (c) the prototype measurement system (A: the system controller; B: pumps for normalization and solution samples; C: sample holder and sensor array; D: low and high normalization solutions; E: drainage tank).
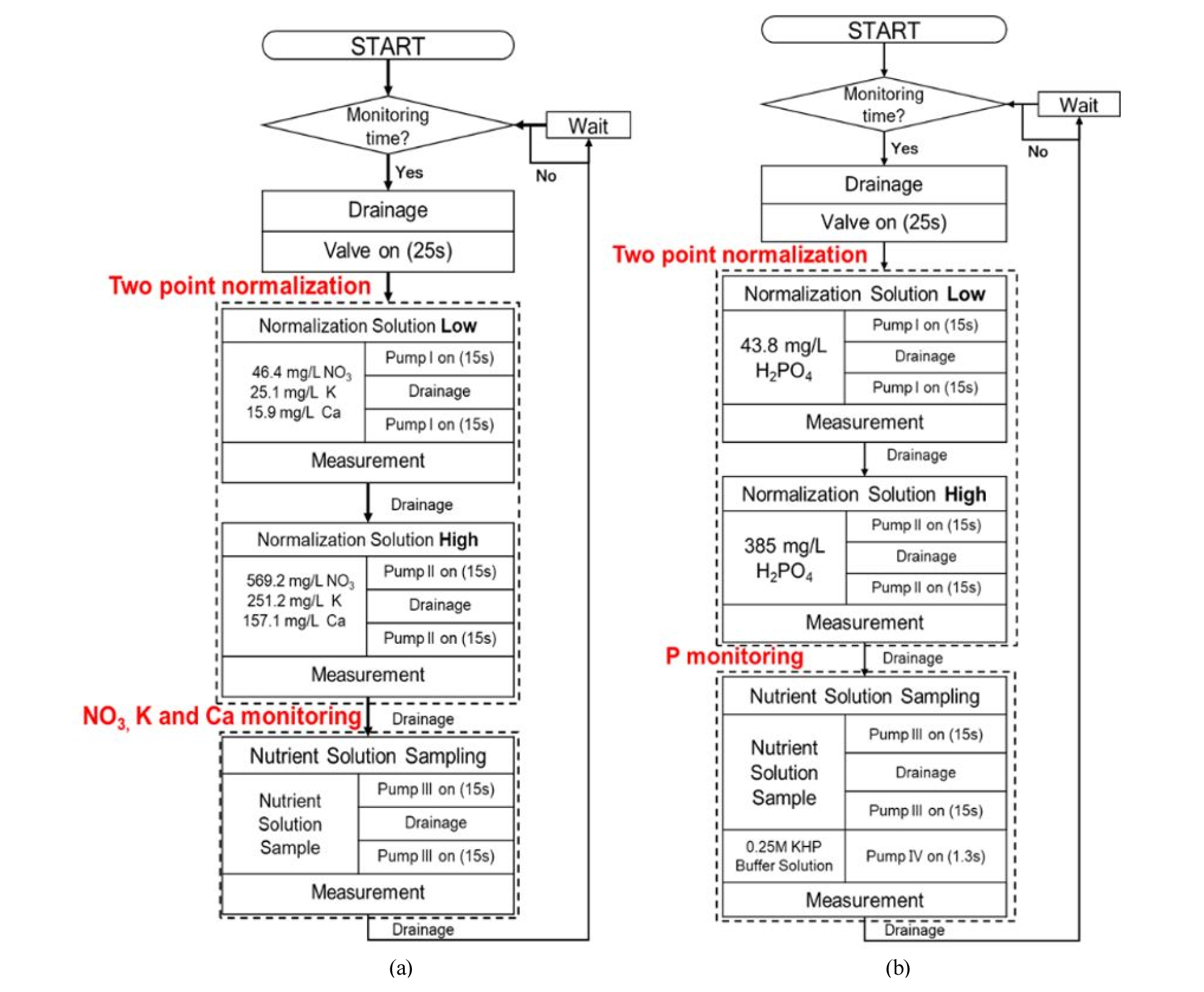
The NO3, K, and Ca ISEs in the sample chamber had a response time of less than 60 s, which was the same as that reported in previous studies (Kim et al., 2013; Jung et al., 2015). Unlike the ISEs for NO3, K, and Ca ions, measurement of the P ions required additional pH stabilization time, so the response time of the cobalt electrodes was 80 s. Analog sensor signals were obtained using a differential amplification circuit consisting of operational amplifiers with a voltage follower and a low-pass filter. The EMF data for the ISEs were stored on an SD card installed on the Arduino Due board. The Arduino board facilitated the control of the volume of the hydroponic solution, and the measurement time and delay were measured for each sample under steady-state conditions. An automatic measurement process was designed in conjunction with two-point normalization of the NO3, K, Ca, and P ions using low and high normalization solution tanks (Fig. 3).
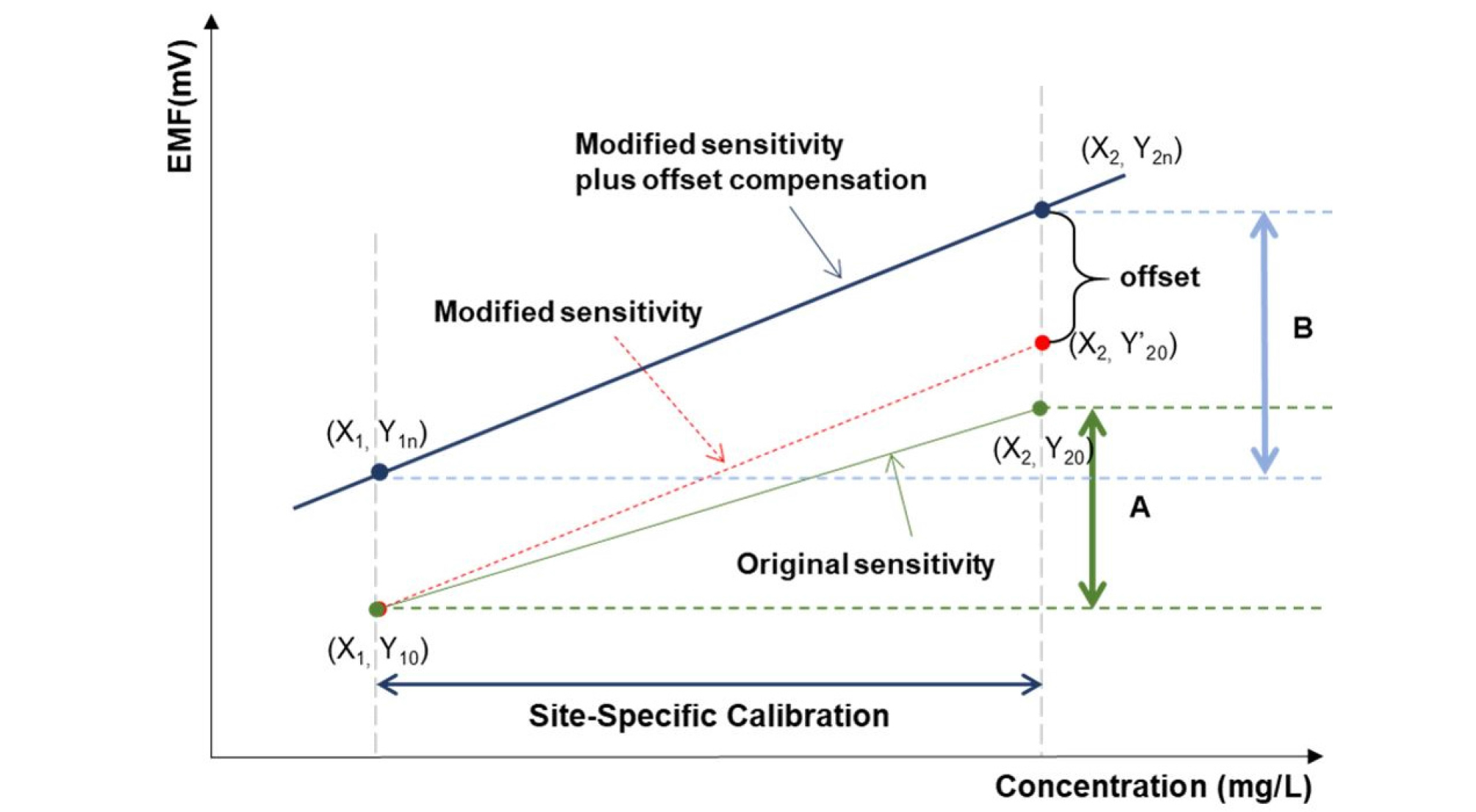
Two-point normalization was employed based on sensitivity compensation followed by an offset to minimize EMF signal drift. As reported by previous studies, three different calibration equations were used to standardize the responses of the electrodes. The two-point normalization equations for NO3 and K ions were taken from Jung (2015), and the equation for Ca ions was taken from Cho (2017). As shown in Fig. 4, two standard samples (low and high normalization solutions) with accurate concentrations were measured and the resulting calibration curve used to compensate for the effect of interfering ions on the slope and intercept. Drift can be eliminated using the two-point normalization of the measured EMF with each sample following Equations (1) – (3). Two-point normalization was conducted just before sample measurement to minimize changes in sensor sensitivity due to temperature (Kim et al., 2013).
| $$\mathrm{ratio}=\frac BA=\frac{Y_{2n}-Y_{1n}}{Y_{20}-Y_{10}}$$ | (1) |
| $$\mathrm{offset}=Y_{2n}-Y'_{20}$$ | (2) |
| $$\mathrm{Compensation}=(\mathrm{EMF}\times\mathrm{ratio})+offset$$ | (3) |
The nutrient solutions were initially prepared following Yamazaki’s (1982) hydroponic formulations for leafy vegetables. Table 2 presents the chemical composition of the fertilizers used in the present study. The low and high normalization solutions were prepared based on the concentrations of NO3, K, and Ca ions in Yamazaki’s hydroponic solution in DI (46 and 569 mg/L for NO3 and 25 and 251 mg/L for K, and 15 and 157 mg/L for Ca, respectively). By adjusting the amount of Ca(NO3)2·4H2O and KNO3 based on Yamazaki’s solution, the sample solutions were prepared to set the NO3, K, and Ca concentrations within the range established by the low and high normalization solutions.
Table 2. Chemical composition of Yamazaki’s (1982) nutrient solutions.
| Solution | Amount (mg/L in DI) | |
| A | Ca (NO3)2·4H2O | 236 |
| Fe-EDTA | 16 | |
| B | KNO3 | 404 |
| MgSO4∙7H2O | 123 | |
| NH4H2PO4 | 57 | |
| H3BO3 | 16 | |
| MnCl2∙4H2O | 0.72 | |
| ZnSO4∙7H2O | 0.09 | |
| CuSO4∙5H2O | 0.04 | |
| (NH4)2MoO4 | 0.01 | |
Fig. 5(b) shows the process for the injection of KHP buffer solution for pH control and the stabilization of the measurement of phosphate ions in the sample solution. To measure the P ions in the sample, 0.25 M KHP buffer solution was diluted 10-fold and mixed with the sample. Due to the low pH (pH 4) of the KHP buffer solution, the NO3, K, and Ca ISE measurements can be affected. Therefore, the metal cobalt electrode measurement process was separated from that for the NO3, K, and Ca ions using the same embedded system. The measurement system was thus programmed to proceed in each sequence NO3, K and Ca ions, and then P ions separately.
The feasibility of the developed system for the measurement of NO3, K, Ca, and P ion concentrations in hydroponic solutions was assessed using hydroponic solution samples. Five samples with different concentrations of the three ions (NO3, K, and Ca) were measured using the prototype monitoring system with ISE data obtained from the three replicates.
Prior to P sensing, the sensitivity of the cobalt-rod-based electrodes to various phosphate concentrations was evaluated by preparing KH2PO4 solutions with concentrations of 10-6 to 10-1 M titrated to pH 4 with 0.025 M KHP based on Yamazaki’s solution. The two-point normalization equation was then obtained in the same way as described above for the three-ion measurement method. Prior to sample measurement, two-point normalization was conducted in the same way using the low and high solutions prepared with 0.025 M KHP (43.8 and 385 mg/L H2PO4 for the low and high concentrations, respectively) to determine the slope and offset of the electrodes. As described above, KHP solution was prepared to measure the P ions and to maintain a steady-state of P ions in the hydroponic solution.
Five sample solutions from real hydroponic systems were tested; A (a hydroponic solution used to grow kale (Brassica oleracea var. sabellica)), B (a higher concentration of hydroponic solution used to grow paprika (Capsicum annuum L. var. grossum)), C (a hydroponic solution used to grow basil (Ocimum basilicum)), D (a hydroponic solution used to grow basil (Ocimum basilicum)), E (a hydroponic solution used to grow beach silvertop (Glehnia littoralis)). These are listed in Table 3 as A–E, respectively, along with their phosphate concentration.
Table 3. Phosphate ion concentrations of five hydroponic solutions analyzed via ion chromatography.
The results measured using the ISEs were compared with those obtained with a standard analyzer. The measurement error was calculated using Equation (4) by comparing the concentration determined by the proposed system with the actual concentration:
| $$\mathrm{Measurementaccuracyerror}(\%)=\frac{\left(\left|AC\mathit-EC\right|\right)}{AC}\ast100$$ | (4) |
where the measurement accuracy error unit is %, AC is the actual concentration measured using a standard analyzer (mg/L), and EC is the concentration estimated using the proposed ISE-embedded system (mg/L). The actual concentrations were determined in a standard testing laboratory (National Instrumentation Center for Environmental Management [NICEM], Seoul, Korea). The EMF measured for the individual ISEs was normalized using the normalization equations stored on the board and the normalized EMF was employed to estimate the concentrations of the ions in the samples.
Results and Discussion
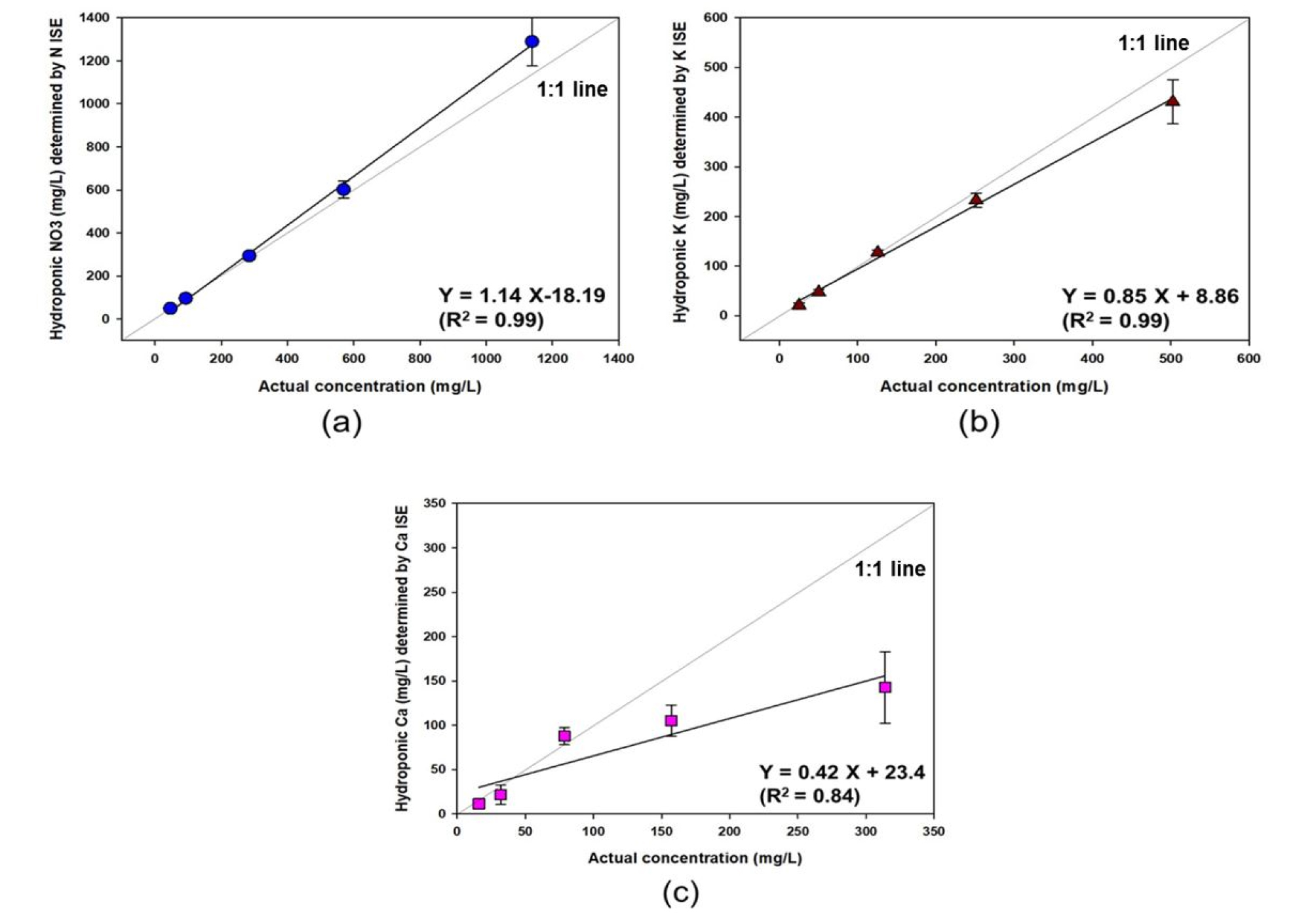
Fig. 6 compares the concentrations of NO3, K, and Ca determined by the proposed system and standard analyzers for the five samples with different concentrations. There was a highly linear relationship between the embedded system and standard analyzers for the measurement of the concentrations of NO3 and K, with slopes close to 1 and a high coefficient of determination (R2) of 0.99, demonstrating the reliability of the ISE-based embedded system in measuring the concentrations of these ions within the range of 50 to 1,000 mg/L in hydroponic solutions. The measurement accuracy error for NO3 and K was 3.7±2.2% to 13.2±9.9%, and 1.0 to 14.0±8.8%, respectively. The regression equations and statistics were calculated using individual data points in Fig. 6.
In contrast, the laboratory-constructed Ca ISE underestimated the Ca concentration, leading to a relatively low coefficient of determination (R2 = 0.84), indicating that Ca ionophore II in the Ca membrane was not suitable for Ca ion measurement at the relatively high concentrations found in hydroponic solutions. These ion measurements were similar to those reported by previous studies (Kim et al., 2017; Cho et al., 2017) using a computer-based embedded system that can be employed as a sensor for field measurement.
Fig. 7(a) compares the EMF response of the three cobalt-rod-based electrodes to various KH2PO4 concentrations ranging from 10-6 to 10-1 M in Yamazaki’s nutrient solution. The cobalt electrodes exhibited a sensitive response to the different KH2PO4 concentrations, with an almost constant slope (35 mV/decade). Fig. 7(b) shows that two- point calibration was obtained within a measurable range above the detection limit (10-4 to 10-1 M), and this was used as the sample calibration curve for the measurement of P ions using the three cobalt electrodes. A sensitivity response with a detection limit in the range of 10-4 to 10-1 M would encompass the typical concentration range found in hydroponic nutrient solutions at agricultural sites.
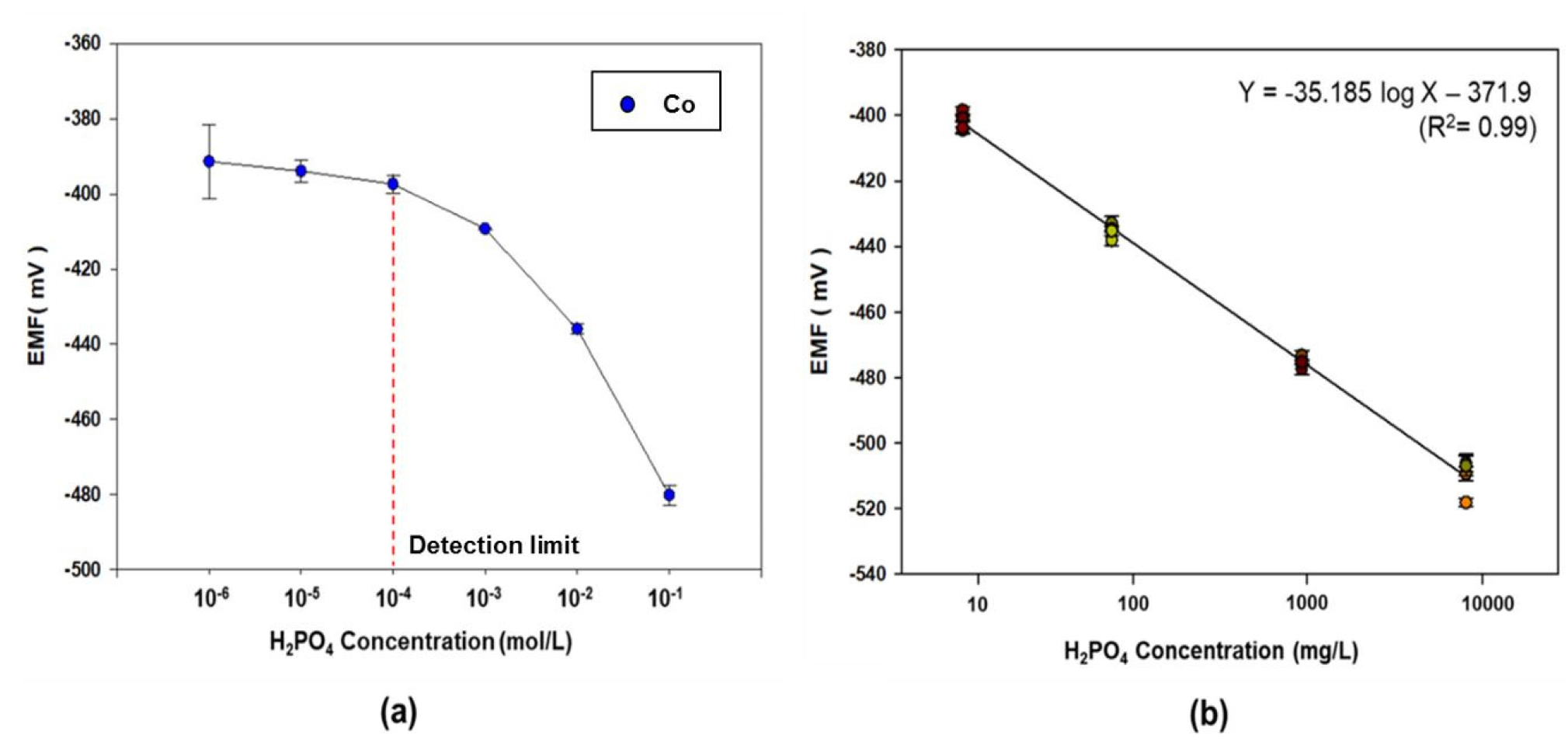
Fig. 7.
(a) Response of the cobalt-rod-based electrodes to different KH2PO4 concentrations (10-6-10-1 M) in Yamazaki’s leaf nutrient solution. (The red dotted line indicates the detection limit, 10-4 M.) (b) the two-point normalization equation obtained for the cobalt electrodes in the range of 10-4 to 10-1 M. The error bars denote the standard deviation in the EMF obtained from the three cobalt electrodes.
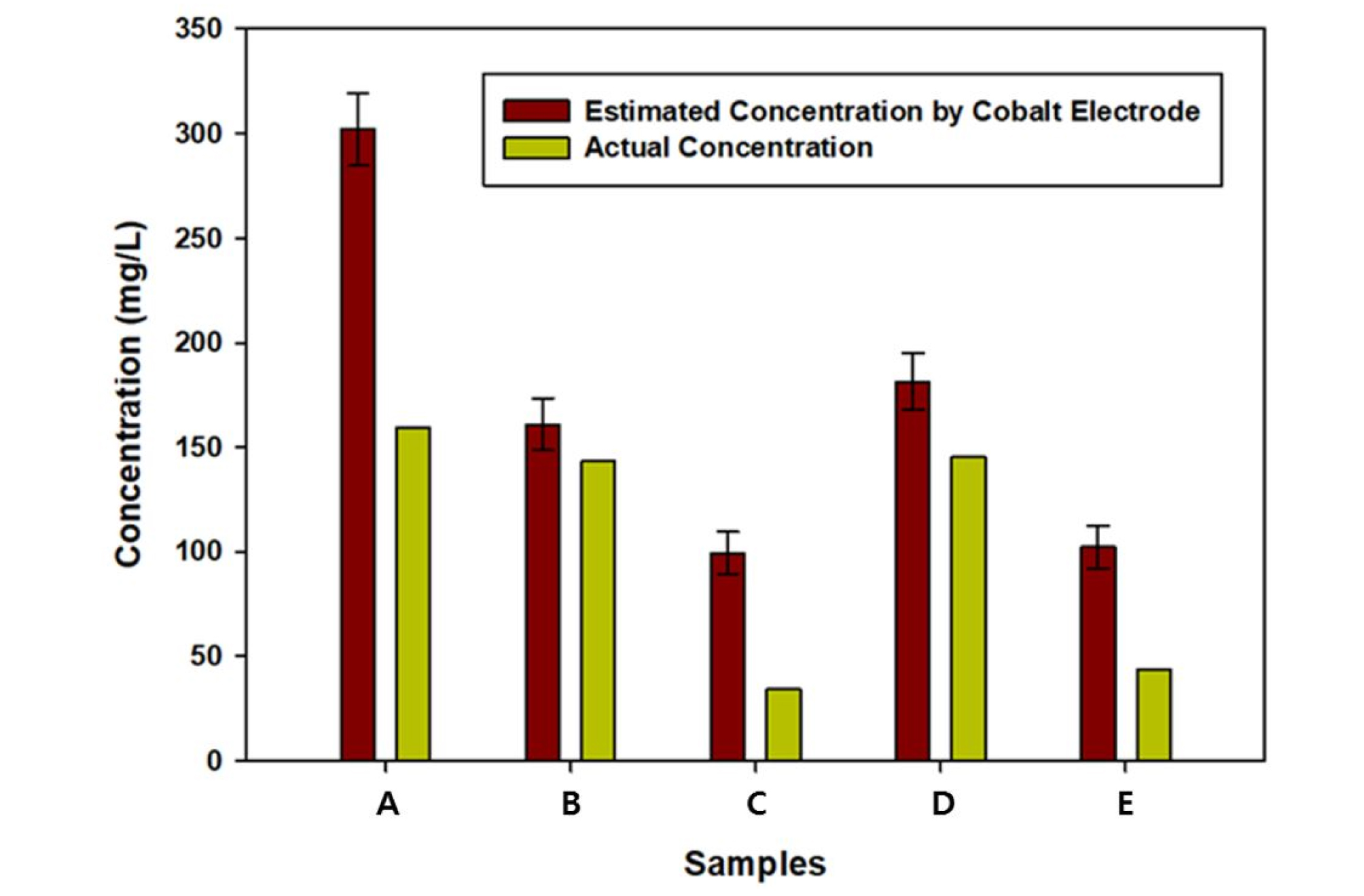
A comparison of the proposed system containing cobalt electrodes with a standard analyzer in the testing of the5 hydroponic solutions is presented in Fig. 8. It can be observed that the estimated P concentrations were not satisfactory overall. However, the measurement accuracy error for the B and D samples were only 12.3±8.7% and 24.7±9.26%, respectively. The error for the C and E samples was over 100%, meaning that the sensitivity of the cobalt electrodes is low at relatively low concentrations. Despite the error rates, the results show that the proposed system could be used to track the trend in P concentration between samples. Unfortunately, sample A had an error close to 100%, indicating high variability in measurement, possibly due to the unstable response of the cobalt electrode.
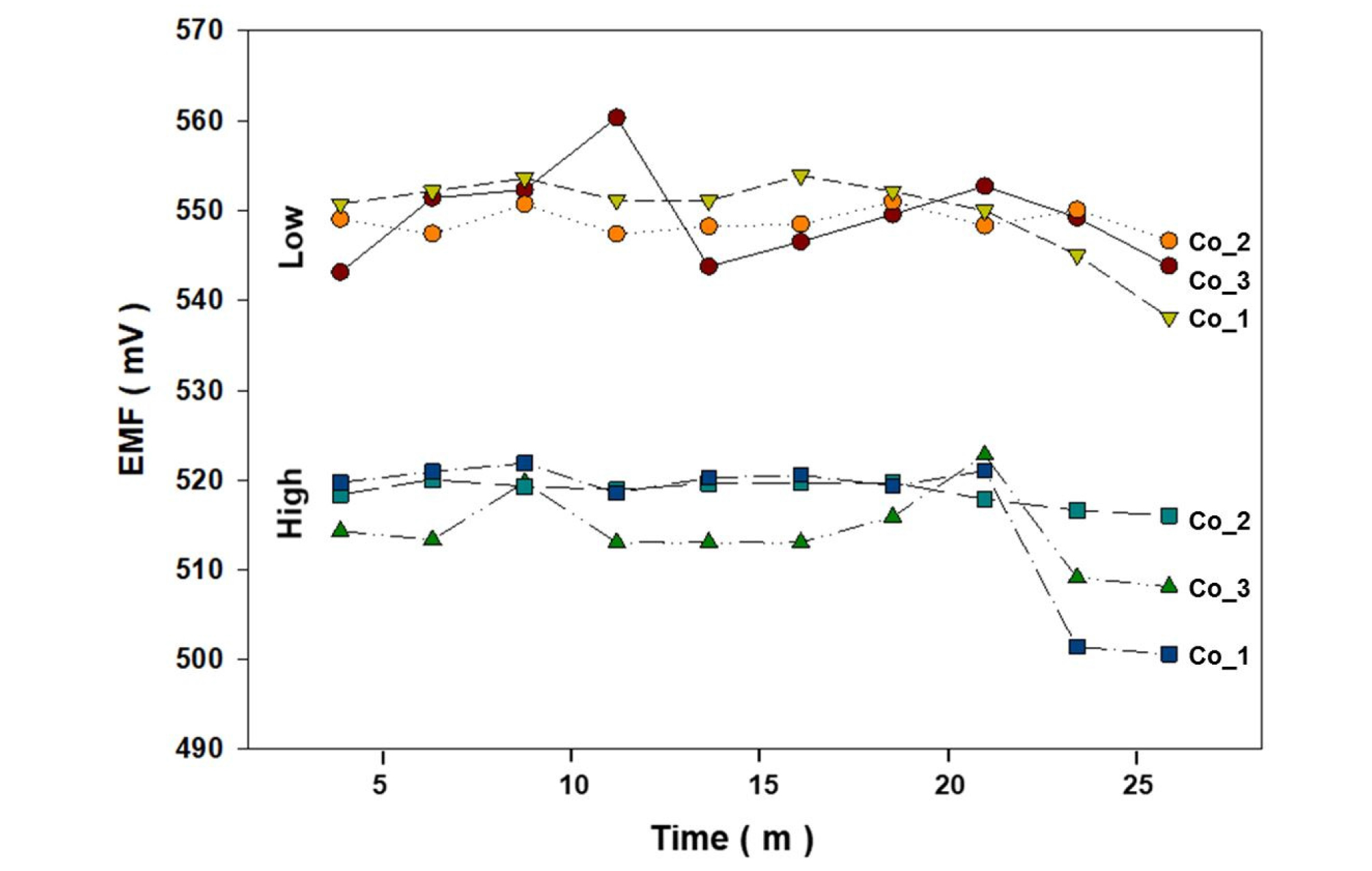
Fig. 9 presents the EMF for the low and high normalization solutions measured twice before the sample measurements shown in Fig. 8. The samples with relatively high measurement error and unstable EMF values were similar for the low and high normalization solution measurements. Future research should be directed toward improving the detection accuracy for P ions in hydroponic solutions and developing a system that can simultaneously measure NO3, K, Ca, and P ions in hydroponic solutions in a more stable manner.
Conclusions
Using an ISE-based embedded system based on autosampling and normalization, it was possible to measure the concentrations of NO3, K, Ca, and P ions in hydroponic solutions in real-time. The predictive capability of the NO3 and K ISEs was satisfactory, producing results that were in close agreement with the results of a standard analyzer. Although the sensitivity of the Ca ISE based on Ca ionophore II was lower at high concentrations, underestimating Ca concentrations by 55% and exhibiting a relatively low coefficient of determination, the results were comparable to the PC-based system developed in a previous study, indicating the possibility of employing the proposed system, with its more compact size, in agricultural fields. Future work will attempt to enhance the sensitivity of the Ca ISE using new material as the Ca ionophore to obtain higher accuracy at high concentrations. The measurement performance of the cobalt electrodes for P ions was relatively stable when measurements were taken in triplicate. However, they were only able to identify the general trend in the concentration of P in real hydroponic solution samples. As a result, this system needs to improve the signal acquisition of the cobalt electrodes in order to enhance P ion measurement performance.